

21 CFR Part 11 is the part of Title 21 of the Code of Federal Regulations that establishes the United States Food and Drug Administration (FDA) regulations on electronic records and electronic signatures (ERES). Part 11, as it is commonly called, defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records.
To enable and to comply with the FDA’s 21 CFR Part 11 (electronic data rule) rule Stability Automation has developed a truly original and cost-effective programmer’s toolkit. This allows 21 CFR Part 11 functionality to be easily incorporated into your application.
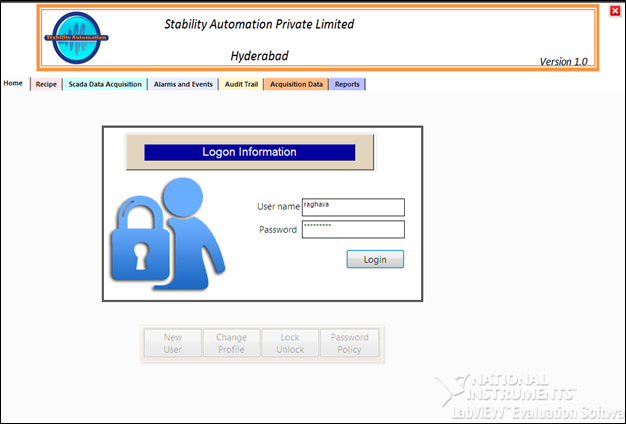
Every Industry need a Security to maintain their records according to the Food & Drug Administration FDA’s 21 CFR Part 11. But maintaining a written record creating a lot of changes in the data and keeping it in safe place becomes problem as they don’t have any backup files once the record sheets lost.
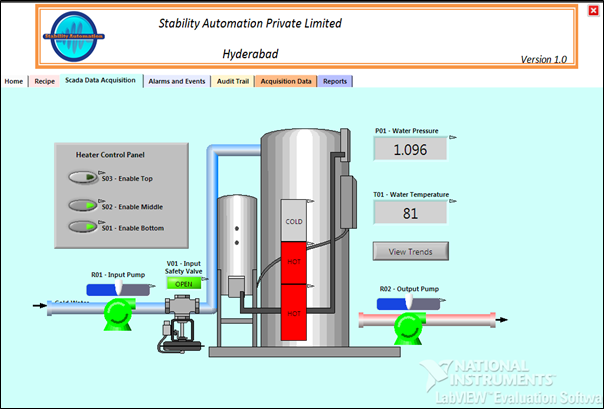
To enable you to comply with the FDA’s 21 CFR Part 11 (electronic data rule) rule Stability Automation has developed a truly original and cost-effective programmer’s toolkit. This allows 21 CFR Part 11 functionality to be easily incorporated into your application.
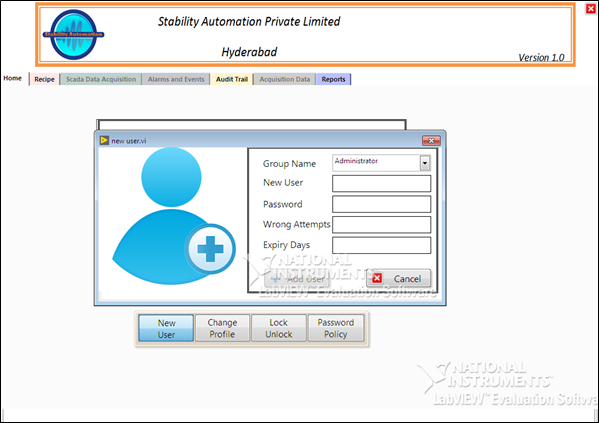
Main components of 21 CFR Part 11 are listed below,
+91 9030070085
+91 7801063999
#501C, Bankers Chambers,
A S Raju Nagar, Kukatpally,
Hyderabad Telangana -500 072.