Executive Summary
Product quality, regulatory compliance and operational efficiencies top the long list of business priorities for pharmaceuticals executives, who lead the charge in improving manufacturing and supply chain performance. Investments in areas such as shop-floor automation, electronic batch records, ERP, and warehouse management systems (WMS) have been made in part to address these concerns, with varying degrees of success. But due to the challenges of batch manufacturing, especially when it comes to monitoring the logistical journey of pharmaceuticals, industry players continually face issues related to product degradation and recalls, compliance with best practices for manufacturing (GMP) and distribution (GDP) – not to mention exorbitant operational costs.
The Internet of Things (IoT), the global network of small, powerful sensors and interconnected “things”, allows physical objects – from heavy equipment, to mobile and wearable devices, to vehicles, physical structures, and appliances – to connect, interact and share data with one another or with a central system through the Internet. This affords higher levels of efficiency, accuracy, and security by digitizing and optimizing critical functions, including manufacturing and supply chain management. According to IDC, there were 9.1 billion IoT units installed in 2013 – a number that IDC predicts will increase to 28.1 billion by 2020.
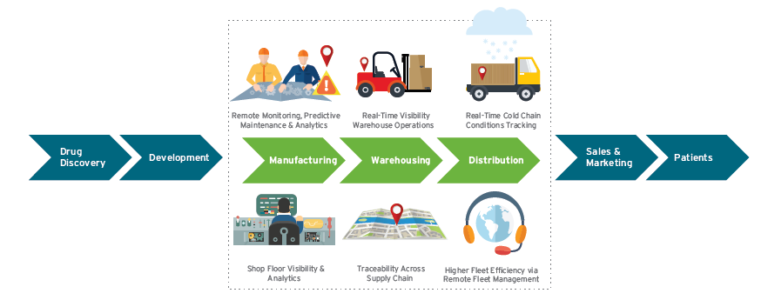
There are multiple scenarios in pharma manufacturing and supply chain management where the Internet of Things can help clear bottlenecks, ensure greater GMP/GDP compliance, and reduce operational expenses.
This white paper explores how pharmaceuticals companies can employ the Internet of Things to automate and revitalize manufacturing and supply chain management. We also present an IoT framework and best practices for accelerating the transition to digital.
Connecting Opportunities Across the Pharma Spectrum
In an IoT environment, every “thing” is equipped with a sensor that allows it to intelligently communicate and interact with other objects and systems within the IoT ecosystem. Each object represents anode in the virtual network – continually transmitting
vital data pertaining to such things as environmental and equipment conditions.
These capabilities are transforming peoples’ lives, and empowering the competitive forces that drive business. Consider the automotive
industry’s focus on connected cars, and health care providers’ enthusiastic adoption of wearable devices. As organizations across sectors examine and evaluate how the IoT can improve how they
manufacture products, provide services, and manage their supply chains, the pharma industry has a compelling opportunity to adopt and profit from these game-changing technologies.
IoT in Pharma Manufacturing/ Supply Chain Management
IoT solutions such as wearable and mobile products have enabled healthcare professionals to make significant inroads in subject/patient health monitoring. The use of intelligent pills2 in
clinical trials is among the applications gaining traction.
IoT applications for manufacturing and supply chain management have become popular investment areas for many industries.
Connected equipment, men and material tracking, sample life cycle management, smart packaging, and cold chain monitoring are among the IoT applications that are particularly well suited to the pharmaceuticals industry
Connected Equipment
Pharmaceuticals manufacturing is generally performed in batches, and equipment is usually self-contained. Although industrial automation and control technologies are well established in life-sciences manufacturing facilities, integrated information on the real-time status or condition of equipment is still not readily accessible to help executives make informed decisions and improve
overall equipment effectiveness (OEE) in areas such as scheduling batches, cleaning, maintenance, etc. These issues point to the shortcomings of current industry solutions.
Because pharma companies rely heavily on batch manufacturing they must constantly move men and material on the shop floor. At the same time, CGMP dictates that equipment be continually
maintained and calibrated to assure the safety and efficacy of drug products. Some raw materials, as well as finished products, require
very specific storage conditions. IoT technologies allow companies to connect and extend visibility into shop-floor activities, which can significantly increase productivity and assure GMP compliance (see Figure 2,).
The backbone of such a solution is made up of sensors attached to manufacturing equipment. The sensors collect and send data to a central system that connects the sensors, then analyzes and converts the raw data into meaningful information on such things as performance and conditions.
Increasing Shop Floor Visibility

Using sensor information to remotely monitor equipment allows technicians to develop self-learning predictive models and perform
diagnostic services across the company’s equipment portfolio – increasing availability and reliability and ensuring minimal disruptions in the supply chain. (See Figure 3 below).
The ability to gather real-time information about equipment availability, calibration and utilization can help manufacturing teams make better decisions, optimize equipment performance,
reduce machine downtime and improve resource allocation, which can lower manufacturing costs and shorten cycle times.
The true value of connected manufacturing varies among companies, since many are at different stages of automating manufacturing. (For more on this topic, read “How Digital Makes Connected Manufacturing Possible.”) The IoT provides a non-linear opportunity to scale by focusing on specific business outcomes.
The biggest challenge is connecting industrial
equipment to sensors or devices to access back-end
Sensor-Based Equipment Monitoring
databases or cloud services. Many companies still operate legacy equipment that does not support standard communications protocols. Pharma companies will thus need to invest in outfitting
existing equipment, or acquire new manufacturing systems to support IoT instrumentation and the digitization of manufacturing and the supply chain. This requires open automation software
that is compatible with a wide range of communication protocols and ensures a seamless transition to the industrial IoT.
Real-Time Visibility Into Warehouse Operations
Warehousing is a crucial area for the pharmaceuticals industry. Most companies manage a large number of storage facilities across countries to ensure a continuous and timely supply of essential medicines in a cost-efficient manner. Keeping warehouse operations in-house is a strategic decision for most pharma companies, many of which choose to manage their warehouse processes and storage environments internally, given the nature of their products.
Nonetheless, warehousing operations is a costly business; according to a McKinsey study,3 it accounts for 95% of pharma logistics costs.
Without real-time visibility into operations, it can be difficult to track products in the warehouse and fully utilize operators and transport equipment.
Smart warehouses can increase visibility and efficiency by relaying metrics and real-time data to warehouse managers and technicians. Sensors are placed in the storage area and on inventory items to interpret and transmit vital information (product location, inventory details) and report inconsistencies, such as misplaced products,directly to warehouse managers’ handheld devices/
dashboards. Corrective measures take place in real time – significantly improving the speed, accuracy, and efficiency of the picking process.
Having real-time visibility and a 3-D view of warehouse operations with detailed, contextually relevant data at their fingertips allows warehouse managers to:
Another area where IoT technologies can add value is the storage of temperature-sensitive products. Active and passive temperature loggers attached to refrigerators in warehouses and at other sites continuously record temperature. An IoT solution could connect these devices, compare their measurements against thermo-stability tables, and prompt them to generate alerts in case of temperature variances.
Sensors can also be used to analyze inventory movement. Data pertaining to the volume and dimensions of packages transmitted from smart pallets can be captured by wireless readers, aggregated, then sent to the central Warehouse Management System (WMS) for analysis.
Figure 4 illustrates how sensor data from warehouse storage areas can be used to dispatch inventory to vacant spaces or re position
misplaced items.
Real-Time, 3-D Inventory Management
Tracking Products in the Warehouse

Track Shipments Across the Pharma Supply Chain Pharmaceuticals packaging is critical to ensuring products quality. Companies must follow strict guidelines for determining the way a drug is transported, administered, and consumed. Smart pharma packaging can help ensure that shipments and medications are accurately
tracked, and that the supply chain remains fluid, efficient and cost-effective.
When applied to packaging, the Internet of Things affords several advantages, including bidirectional communication, tracking, and status display mechanisms. This is especially relevant in markets where counterfeit drugs regularly enter the value chain. Tracking the movement of drug inventory at every point can potentially save
supply chain participants billions of dollars.
2-D bar codes, RFID tags and smart packaging labels make it possible to track each handshake in the supply chain, from manufacturing to dispensing. The result is a complete digital footprint. Electronic circuits/chips in packaging material and the use of Auto-ID with Automatic Information Data Collection (AIDC) for smart serialization can help control how substances enter healthcare environments and ensure that suitable conditions are maintained in cold chains during the transport of temperature-sensitive drugs. (See Figure 6).
Tracking Products at Every Point

Control Cold Chain Conditions
Pharmaceuticals manufacturers increasingly deal with products such as biologics, which are highly sensitive to storage conditions. These are high-ticket drugs. Last year alone, eight of the top ten drugs (measured by highest sales) were biologics. Moreover, the projected growth of cold-chain biopharma products will be twice that of the industry overall – expected to reach more than $361 billion worldwide in 2019.
Monitoring Cold Chain Conditions on Shipping Vehicles
Managing Temperature Spikes
These products often have a larger proportion of high-value active ingredients with shorter shelf lives, and carry strict temperature requirements. Many must be maintained at temperatures lower than 77 degrees Fahrenheit; some require 35 to 46 degree Fahrenheit during cold chain transportation. Quite a few must be stored at controlled room temperature. These drugs are safe at ambient temperatures, but must be kept in temperature-controlled containers during transport, like reefers, to avoid the spikes that
can occur in ambient containers.
Yet pharma companies continue to lose millions due to spoilage from temperature fluctuations, and face an even greater threat if the deterioration goes unnoticed – leading to potential health hazards for patients and subsequent regulatory actions. This issue is increasingly critical in the emerging Asian and South American markets, where wide temperature fluctuations are more the rule than the exception.
The existing cold chain logistics and storage market handles approximately $260 billion worth of products;6 it is estimated that 20% of these goods are wasted during shipping and transportation. IoT-based solutions can help pharma manufacturers remotely monitor cold chain environments in real time by embedding sensors on tracking equipment with auto-start and shut-down mechanisms in warehouses, vehicles, or shipments using smartphones and
tablets. (See Figures 7, and Figure 8, above).
Remote Fleet Management
According to the 2013 Global Cargo Theft Threat Assessment 7 from the Freight Watch International Supply Chain Intelligence Center, the pharmaceuticals industry has experienced some of the highest average loss values due to cargo theft. The same report states that the average load loss for the pharma sector in the U.S. has dropped dramatically – from $3.78 million in 2010 to $168,219 in 2012 – due to the industry’s adoption of sophisticated methods for freight
tracking and data collection. This assumes that by taking a similar approach to ensuring shipment visibility throughout the supply chain, pharma manufacturers can initiate real-time tracking of cargo conditions and vehicle performance, and avoid losses and penalties from late deliveries and spoilage.
Digital technologies such as GPS location and condition monitoring afford real-time visibility and security during transport.
Generics make up 80 percent of today’s pharma market and competition is fierce. This underscores the importance of developing transport and logistics capabilities that heighten efficiencies and drive down costs.
Digital technologies such as GPS location and condition monitoring afford real-time visibility and security during transport. Figure 9
illustrates how pharma manufacturers can unify the view of vehicles, drivers, and performance across locations by collecting data from vehicle telematics. This information can be captured via
Global Positioning Systems (GPS), then relayed to control rooms through GPRS (General Packet Radio Service) – allowing complete visibility into product and transport conditions, and enabling control-room personnel to analyze and compare data on fleet performance from virtually anywhere. This can reduce time to market, prevent product damage and waste, and lower carrying costs.
Intelligent Fleet Management Using GPRS & Sensors
Intelligent Fleet Management Using GPRS & Sensors
IoT Adoption: The Challenges
To fully capitalize on the potential of the Internet of Things and enjoy the benefits throughout their value chain, pharma companies must first invest in a supportive IoT infrastructure with the capacity needed to handle heavy-duty requirements. Since security is paramount, companies must also be prepared to invest in IoT-based
security solutions.
In cases that require integration with underlying applications, working with various vendors and ensuring common vocabularies and standards can be challenging. Moreover, the manufacturing
floor typically houses a broad spectrum of equipment and associated software, some of which, while fine for production purposes, are no
longer under warranty by vendors. Workarounds for IoT solutions can be costly.
Another hurdle pertains to the availability and suitability of manufacturing equipment to modify and validate IoT solutions. Balancing project requirements with commercial supply needs and
key performance indicators (KPIs) for gauging operational efficiency can be overwhelming. The IoT solution may require validation if its purpose is to support GMP best practices. This could extend project timelines and efforts.
IoT solutions such as shop-floor visibility augment and enrich organizational capabilities. At the same time, they can present challenges when it comes to change management – a sensitive issue that must take into account people, processes, and responsibilities. Organizations need to establish and clarify business drivers and develop communication strategies for assuaging the concerns of employees and other key stakeholders to ensure a successful
transition.
Pharma companies must view their IoT investments from the standpoint of fostering innovation, heightening efficiencies, improving processes and operational performance, and adding more value for the business, its suppliers, and its customers. By adhering to IoT best practices and learning from others that have
successfully implemented IoT platforms and solutions, pharmaceuticals companies can be better prepared to face the growing demands of today’s hyper-competitive, hyper-connected
global economy. (See Figure 10).
Recommendations
Investing in transformational technologies has its own set of challenges. Following are our recommendations and best practices for implementing and benefiting from the Internet of Things.
Looking Ahead
In virtually every industry, advances in digital technologies are redefining how enterprises conduct business – internally, with external stakeholders, and in the marketplace. The ubiquity of mobile computing, the dominance of social media, and a growing portfolio of smart products and capabilities provide real-time, actionable intelligence at a pace that continues to increase. Enterprises must constantly innovate and utilize emerging technologies to remain relevant, competitive, and profitable.
For pharmaceuticals companies, the Internet of Things extends visibility in virtually every area of the business – from development, to manufacturing, transport, distribution, dispensing and consumption. Real-time information, when coupled with advanced analytics engines, can become the basis for making faster, more accurate decisions; heightening efficiencies, verifying product quality, and assuring regulatory compliance.
As the IoT becomes mainstream, it presents an opportunity to digitize business processes and enable collaboration and monitoring beyond what was possible before now. The risk of doing nothing
must be evaluated against changing customer and regulatory expectations and market dynamics. The time is right for pharmaceuticals companies to accelerate their implementation and utilization of IoT platforms and solutions.